
OPC元 is bonded to four groups making it a tetrahedral molecule. This can only be observed from the structure of the compound.

How ever, there are two lone pairs in the molecule hence it is bent.ĬH2Cl2 is shows a tetrahedral molecular geometry and a tetrahedral electron geometry.
ELECTRON AND MOLECULAR GEOMETRY CALCULATOR HOW TO
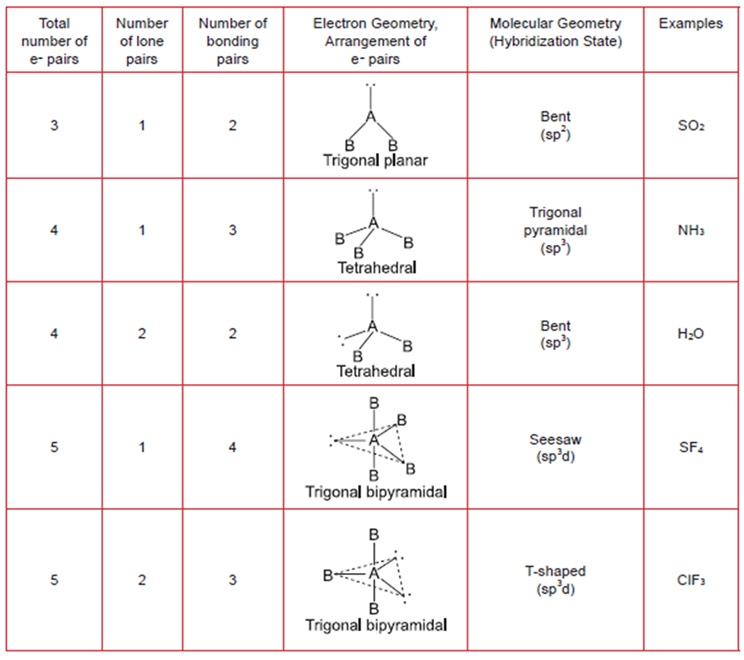
How to Determine Molecular Geometry - YouTubeThis video describes one method for quickly finding the major geometrical shapes for simple molecules.Water contains four electron domains this corresponds to a tetrahedral electron geometry. VSEPR table of molecular geometriesThe bonded angles in the table are ideal angles from the simple VSEPR theory the actual angle for the example given is in the following column. VSEPR geometriesA visual guide to molecular geometries using the VSEPR Theory. The second figure serves as a visual aid for the table. The table of molecular geometries can be found in the first figure. A table of geometries using the VSEPR theory can facilitate drawing and understanding molecules. Molecular geometries (linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral) are determined by the VSEPR theory. If the central atom possesses partially occupied d-orbitals, it may be able to accommodate five or six electron pairs, forming what is sometimes called an "expanded octet."
The orbitals containing the various bonding and non-bonding pairs in the valence shell will extend out from the central atom in directions that minimize their mutual repulsions. If the central atom also contains one or more pairs of non-bonding electrons, these additional regions of negative charge will behave much like those associated with the bonded atoms. The two X atoms (in white) are 180° away from one another. Linear electron geometryThis ball-and-stick model represents a linear compound for formula AX2. An angular separation of 180° places the two bonding orbitals as far away from each other as possible we therefore expect the two chemical bonds to extend in opposite directions, producing a linear molecule.

Therefore, the two electron clouds contained in a simple triatomic molecule AX 2 will extend out in opposite directions. The valence shell electron pair repulsion (VSEPR) model focuses on the bonding and nonbonding electron pairs present in the outermost (valence) shell of an atom that connects with two or more other atoms.įundamentally, the VSEPR model theorizes that these regions of negative electric charge will repel each other, causing them (and the chemical bonds that they form) to stay as far apart as possible.


 0 kommentar(er)
0 kommentar(er)
